Introduction
Extracellular metalloproteinases are major players in development and in various physiological and pathological tissue remodeling processes (pregnancy, bone growth, wound healing, atherosclerosis, tumor progression, osteoarthritis etc). Among their important functions, they control the synthesis and degradation of extracellular matrix proteins, the activation and inactivation of growth factors and cell-matrix interactions.
Our group focusses on a subfamily of metalloproteinases, known as the BMP-1/tolloid-like proteinases (BTPs), which were first described for their important role in collagen fibrillogenesis. Indeed, by cleaving the C-terminal propeptides of collagens I, II and III, BTPs trigger collagen fibril formation. They also control the proteolytic maturation of several proteoglycans, cross-linking enzymes, basement membrane proteins and mineralization factors. More recently, it was shown that BTPs regulate the activity of several growth factors (TGF-β, BMP-2/4/11, GDF8, IGF) and have the capacity to synchronize matrix assembly with growth factor activation in order to promote tissue morphogenesis and remodeling. Therefore, they play an essential role in development, bone remodeling and tissue repair. It is also important to note that these proteinases are part of a complex network involving regulatory proteins and other proteinases which can potentially cleave the same substrates, leading to interesting mechanisms of synergy, inhibition or competition.
Our main objective is to better understand the functions of the BTPs and of their main partners (substrates, regulatory proteins, associated proteinases) in the context of tissue repair, in order to propose novel therapeutic strategies to be applied to human diseases such as fibrosis, chronic wounds or corneal scarring. To achieve this, we use a combination of complementary approaches including biochemistry, structural biology, enzyme assays, proteomics, cell biology and animal models.
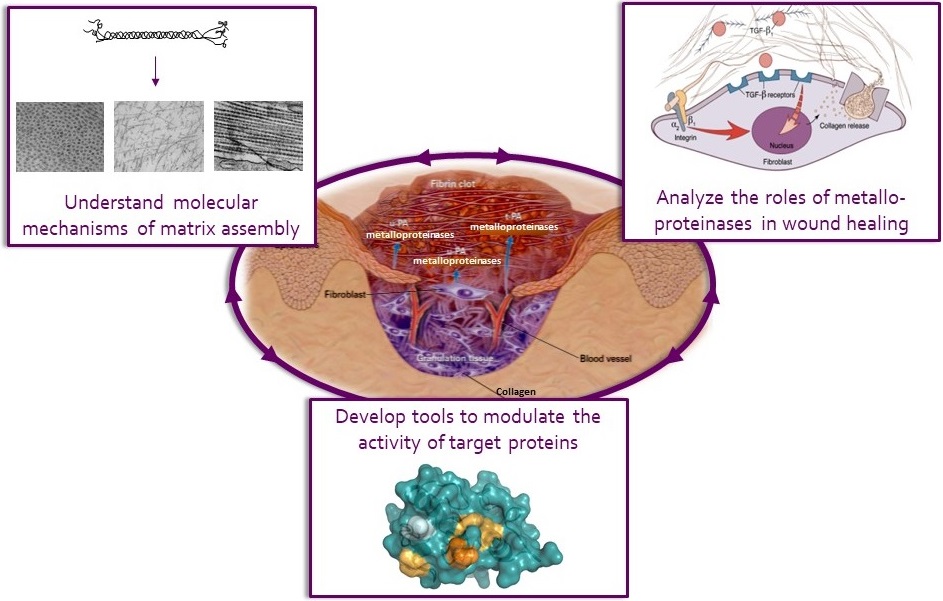
Summary of our main research axes. We study the molecular mechanisms (both proteolytic and non proteolytic) of extracellular matrix assembly and the role of various metalloproteinases in cutaneous and corneal wound healing. In addition, we develop synthetic and protein inhibitors of the most promising therapeutic targets identified in above studies. Pictures adapted from Singer et al. N. Engl. J. Med. 1999, Schultz et al. Wound Repair Regen. 2009 and Binz et al. Nat. Biotechnol. 2004.
Research topics
Our research activity is organized around three main axes :
• Molecular mechanisms of collagen assembly (C. Moali and S. Vadon-Le Goff)
• Role of metalloproteinases and associated regulatory proteins in tissue repair (C. Moali)
• Development of inhibitors and activators of target proteins (S. Vadon-Le Goff)
Keywords :
Metalloproteinases, Collagen, Extracellular Matrix, TGF-β, Fibroblasts, Wound Healing, Skin, Cornea, Inhibitors
Members : Équipe Métalloprotéases et Remodelage tissulaire
Publications : Publications Métalloprotéases et Remodelage tissulaire
Contact
Metalloproteinases and Tissue Remodeling Group
Tissue Biology and Therapeutic Engineering Laboratory – CNRS – UMR 5305 – University Claude Bernard Lyon 1
Institut de Biologie et Chimie des Protéines
7, passage du Vercors 69367 LYON CEDEX 07
FRANCE
E-mail : c.moali@ibcp.fr
Tel : : +33 (0)4-72-72-26-38
Fax : +33 (0)4-72-72-26-04



