Project leader : C. Moali
Wound healing is an example of process which involves extensive remodeling with several steps : hemostasis, inflammation, angiogenesis, re-epithelialization, formation of a provisional extracellular matrix (granulation tissue), wound contraction and matrix remodeling. The BMP-1/tolloid-like proteinases (BTPs) being essential for matrix assembly, we made the hypothesis that they would be strongly up-regulated during the granulation and remodeling phases. This was demonstrated in a model of corneal wound healing and in human corneas collected from patient undergoing corneal transplantation (Figure). We currently characterize other types of repair processes (skin and heart) to determine if this observation can be extended.
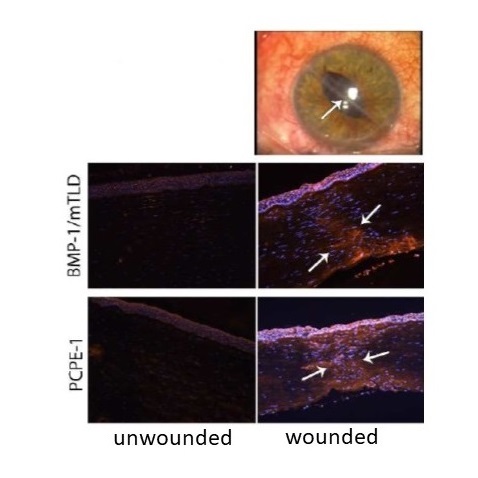
Immunofluorescence detection of the BMP-1/mTLD proteinase and of the regulatory protein PCPE-1 in the area of a central injury of the cornea associated with scarring (D’après Malecaze et al. Invest. Ophthalmol. Vis. Sci. 2014).
In order to better understand the role played by BTPs and associated proteinases (ADAMTS-2, 3 and 14; meprins α and β) in wound healing, we also use quantitative proteomics approaches allowing the identification of proteinase substrates in cell-based models or tissue samples. These techniques have already revealed several novel functions for BTPs and ADAMTSs, especially in the control of TGF-β activity, with major implications for many aspects of the wound healing process (inflammation, synthesis of matrix proteins, wound contraction).
Main techniques used
• Quantitative PCR
• Immuno-fluorescence / western-blotting / ELISA
• Cell biology
• Enzyme assays
• Mass spectrometry and proteomic analysis
Selection of publications :
BMP-1 disrupts cell adhesion and enhances TGF-beta activation through cleavage of the matricellular protein thrombospondin-1. Anastasi C, Rousselle P, Talantikite M, Tessier T, Cluzel C, Bachmann A, Mariano N, Dussoyer M, Alcaraz LB, Fortin L, Aubert A, Delolme F, El Kholti N, Armengaud J, Fournié P, Auxenfans C, Valcourt U, Vadon-Le Goff S, Moali C. Science Signaling 2020; 13(639): eaba3880.
Access to the full-text available here : http://stke.sciencemag.org/cgi/content/full/sigtrans;13/639/eaba3880?ijkey=XTLgOBuiU1o0Y&keytype=ref&siteid=sigtrans
Acess to the reprint : http://stke.sciencemag.org/cgi/reprint/sigtrans;13/639/eaba3880?ijkey=XTLgOBuiU1o0Y&keytype=ref&siteid=sigtrans
C-terminal proteolysis of the collagen VI α3 chain by BMP-1 and proprotein convertase(s) releases endotrophin in fragments of different sizes. Heumüller SE, Talantikite M, Napoli M, Armengaud J, Mörgelin M, Hartmann U, Sengle G, Paulsson M, Moali C, Wagener R. J Biol Chem. 2019;294(37):13769-13780.
Interaction of Complement Defence Collagens C1q and Mannose-Binding Lectin with BMP-1/Tolloid-like Proteinases. Lacroix M, Tessier A, Dumestre-Pérard C, Vadon-Le Goff S, Gout E, Bruckner-Tuderman L, Kiritsi D, Nyström A, Ricard-Blum S, Moali C, Hulmes DJS, Thielens NM. Sci Rep. 2017;7(1):16958.
Determination of the substrate repertoire of ADAMTS2, 3, and 14 significantly broadens their functions and identifies extracellular matrix organization and TGF-β signaling as primary targets. Bekhouche M, Leduc C, Dupont L, Janssen L, Delolme F, Vadon-Le Goff S, Smargiasso N, Baiwir D, Mazzucchelli G, Zanella-Cleon I, Dubail J, De Pauw E, Nusgens B, Hulmes DJ, Moali C, Colige A. FASEB J. 2016;30(5):1741-56.
Proteolytic control of TGF-β co-receptor activity by BMP-1/tolloid-like proteases revealed by quantitative iTRAQ proteomics. Delolme F, Anastasi C, Alcaraz LB, Mendoza V, Vadon-Le Goff S, Talantikite M, Capomaccio R, Mevaere J, Fortin L, Mazzocut D, Damour O, Zanella-Cléon I, Hulmes DJ, Overall CM, Valcourt U, Lopez-Casillas F, Moali C. Cell Mol Life Sci. 2015;72(5):1009-27.
Upregulation of bone morphogenetic protein-1/mammalian tolloid and procollagen C-proteinase enhancer-1 in corneal scarring. Malecaze F, Massoudi D, Fournié P, Tricoire C, Cassagne M, Malbouyres M, Hulmes DJ, Moali C, Galiacy SD. Invest Ophthalmol Vis Sci. 2014;55(10):6712-21



