Project 3A. A molecular signature of severe side effects of radiotherapy. Project leader: J. Lamartine
The skin is a direct target of environmental stresses. The cutaneous sensitivity to stress is highly variable among individuals. The identification of individuals hypersensitive to ionizing radiations is a major issue to prevent the secondary effects of radiotherapy. We are establishing a molecular and epigenetic signature of the cutaneous radiosensitivity using fibroblasts from patients suffering severe side effects of radiotherapy. We also want to better understand the mechanistic basis of this radiosensitivity and propose new diagnosis strategies to identify at risk individuals. This approach can be proposed for other environmental stresses such as climatic changes, air pollution, visible light, chemical pollutants and electromagnetic waves.
Recent publications of the group on this topic:
– Dulong J, Kouakou C, Mesloub Y, Rorteau J, Moratille S, Chevalier F, Vinasco-Sandoval T, Martin MT, Lamartine J. NFATC2 modulates radiation sensitivity in dermal fibroblasts from patients with severe side-effects of radiotherapy. Front Oncol. 2020 Dec 16; 10:589168.
– Vulin A, Sedkaoui M, Sevenet N, Moratille S, Soularue P, Rigaud O, Guibbal L, Dulong J, Jeggo P, Deleuze JF, Lamartine J, Martin MT. Severe PTCH1 expression deficiency in cancer-prone Gorlin cells results in intrinsic radiosensitivity. Int J Radiat Oncol Biol Phys. 2018 Oct 1;102(2):417-425
irradiated skin fibroblasts
Project 3B. Skin neurovascular alteration and risk of pressure ulcers in paraplegic and diabetic patients. Project leaders: B. Fromy & D. Sigaudo-Roussel
Pressure ulcers (PU) and their treatment represent one of the most challenging clinical problems faced by patients who are elderly, diabetic, neurologically impaired or have chronic spinal cord injury (SCI). We previously showed that peripheral sensory neuropathy is associated with PU (Gaubert et al. 2013) and worsened alteration of skin vascular reactivity capacities to pressures in diabetes (Demiot et al. 2006) and aging (Fromy et al. 2010).
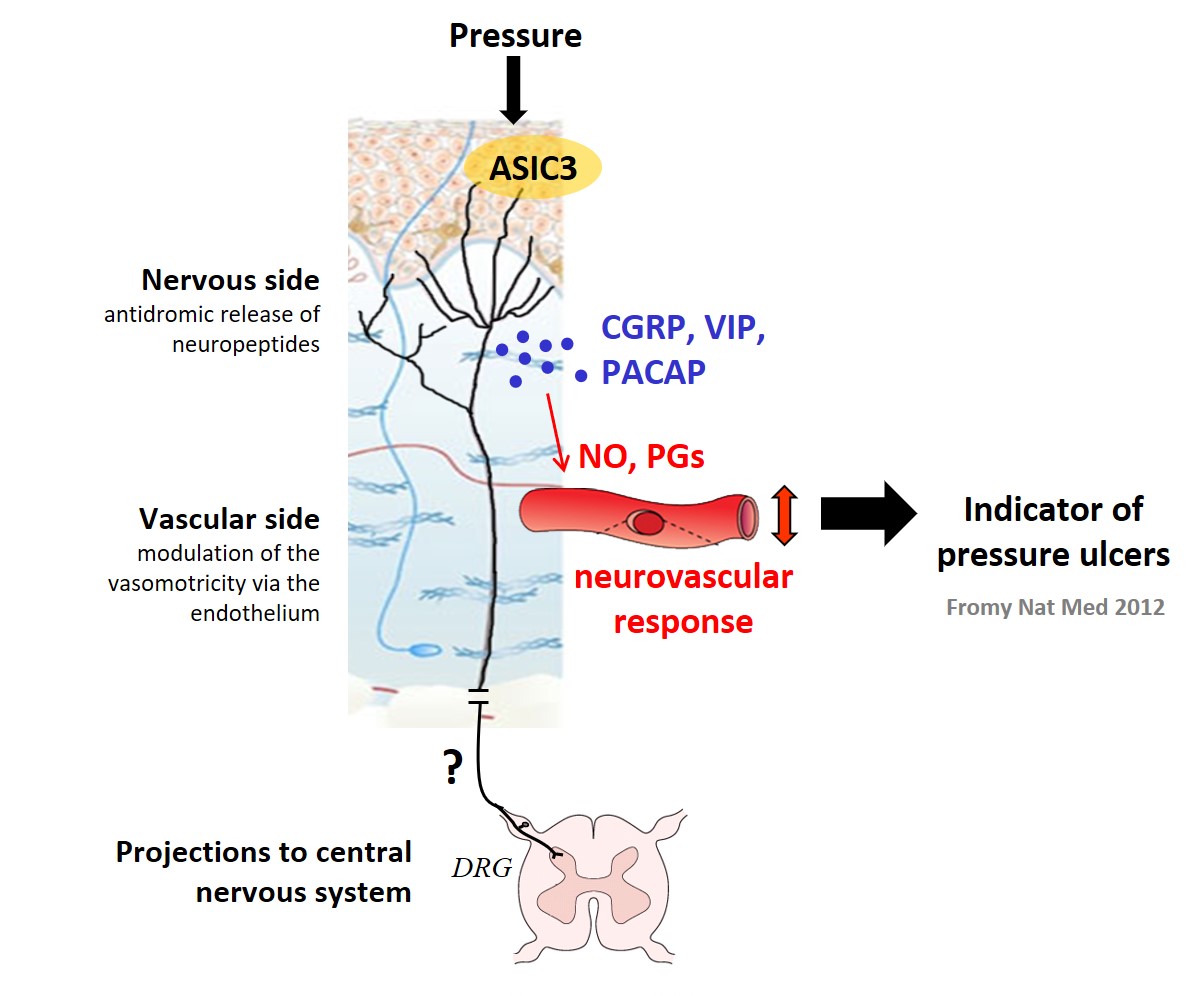
Based on our previous results (Fromy et al. 2012, figure), we hypothesize that the neurovascular capacities of the cutaneous microcirculation are abolished in paralyzed territory (high risk for PU) and preserved in non-paralyzed territory in the complete paraplegic patient. No studies have been done to demonstrate it to date. We aim to investigate this hypothesis in SCI patients and evaluate whether the skin neurovascular alterations will be correlated to the PU risk evaluated by a standard clinical scale of risk.
A pilot study (Vouillarmet et al. 2019) revealed that a pronounced alteration of neurovascular response to pressure in patients with diabetic foot ulcer (DFU) is a good marker of skin vulnerability and could be used to better predict individuals at risk. We are currently pursuing this study to validate this hypothesis by a 3-year follow-up of diabetic patients without history of DFU at the time of recruitment and correlating the neurovascular response to the occurrence of DFU.
Publications of the group on this topic:
Vouillarmet J, Josset-Lamaugarny A, Michon P, Saumet JL, Koitka-Weber A, Henni S, Fromy B, Sigaudo-Roussel D. Neurovascular response to pressure in patients with diabetic foot ulcer. Diabetes 2019 ; 68(4):832-836.
Gaubert-Dahan ML, Castro-Lionard K, Blanchon MA, Fromy B. Severe sensory neuropathy increases heel pressure ulcer risk in older adults. J Am Geriatr Soc 2013; 61(11): 2050-2052
Fromy B, Lingueglia E, Sigaudo-Roussel D, Saumet JL, Lazdunski M. Asic3 is a neuronal mechanosensor for pressure-induced vasodilation that protects against pressure ulcers. Nature Medicine 2012 ; 18: 1205-1207



