Project leader : M. Ducret
Participant : M. Bekhouche, J-C Farges, M. Pasdeloup, M. Lévêque
Infections of teeth are a major concern of public health worldwide. Two strategies are proposed to regenerate dental pulp: a « cell-based strategy » uses exogenous cells and a biomaterial (i), and a « cell-free strategy » allows host cells to colonise a biomaterial to reconstruct a new dental pulp (ii).
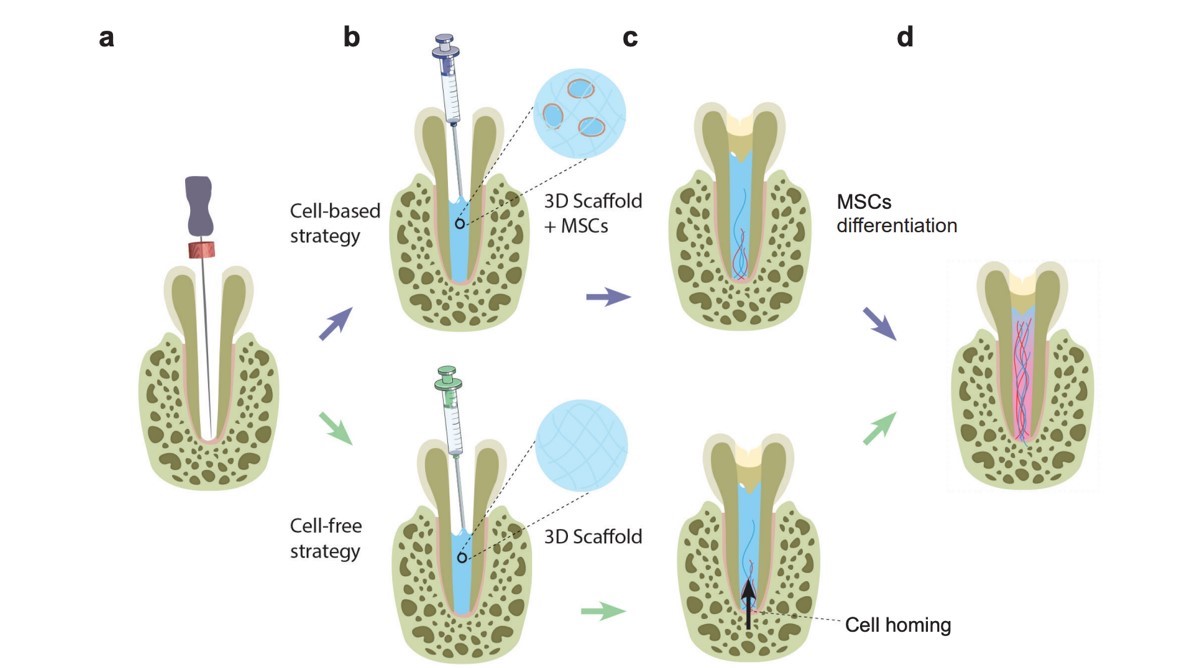
Figure 1 : Illustration of two strategies for dental pulp reconstruction.
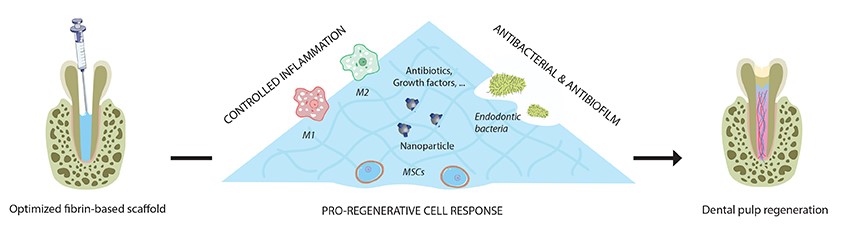
Figure 2 : Illustration of different axes of the project ENDONANOBIOTIC
Selected publications :
Development of an antibacterial nanocomposite hydrogel for human dental pulp engineering. J Mater Chem B. 2020 Sep 23;8(36):8422-8432. doi: 10.1039/d0tb00989j. PMID: 32804177
– Ducret M, Farges JC, Pasdeloup M, Perrier-Groult E, Mueller A, Mallein-Gerin F, Fabre H. Phenotypic Identification of Dental Pulp Mesenchymal Stem/Stromal Cells Subpopulations with Multiparametric Flow Cytometry.
Methods Mol Biol. 2019;1922:77-90. doi: 10.1007/978-1-4939-9012-2_8.
– Ducret M, Montembault A, Josse J, Pasdeloup M, Celle A, Benchrih R, Mallein-Gerin F, Alliot-Licht B, David L, Farges JC. Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. Dent Mater. 2019 Apr;35(4):523-533. doi: 10.1016/j.dental.2019.01.018. Epub 2019 Jan 31.
– Ducret M, Fabre H, Celle A, Mallein-Gerin F, Perrier-Groult E, Alliot-Licht B, Farges JC. Current challenges in human tooth revitalization. Biomed Mater Eng. 2017;28(s1):S159-S168. PMID: 28372291. doi: 10.3233/BME-171637.
– Ducret M, Fabre H, Degoul O, Atzeni G, McGuckin C, Forraz N, Mallein-Gerin F, Perrier-Groult E, Alliot-Licht B, Farges JC. Immunophenotyping Reveals the Diversity of Human Dental Pulp Mesenchymal Stromal Cells In vivo and Their Evolution upon In vitro Amplification. Front Physiol. 2016 Nov 8;7:512. eCollection 2016. PMID: 27877132. doi: 10.3389/fphys.2016.00512
– Park S-H, Ye L, Love RM, Farges J-C, Yumoto H. Inflammation of the Dental Pulp. Mediat Inflamm 2015; Article ID 980196. doi: org/10.1155/2015/980196.
– Ducret M, Fabre H, Degoul O, Atzeni G, Mcguckin C, Forraz N, Alliot-Licht B, Mallein-Gerin F, Perrier-Groult E, Farges J-C. Manufacturing of dental pulp cell-based products from human third molars : current strategies and future investigations. Front Physiol 2015 ;6:213. doi:10.3389/fphys.2015.00213.
– Ducret M, Fabre H, Farges J-C, Degoul O, Atzeni G, Mcguckin C, Forraz N, Mallein-Gerin F, Perrier-Groult E. Production of human dental pulp cells with a medicinal manufacturing approach. J Endod 2015 ; 41(9):1492-9. doi : 10.1016/j.joen.2015.05.017.
– Farges J-C, Bellanger A, Ducret M, Aubert-Foucher E, Richard B, Alliot-Licht B, Bleicher F, Carrouel F. Human odontoblast-like cells produce nitric oxide with antibacterial activity upon TLR2 activation. Front Physiol 2015 ;6:185. doi : 10.3389/fphys.2015.00185.
– Gaudin A, Renard E, Hill M, Bouchet-Delbos L, Bienvenu-Louvet G, Farges J-C, Cuturi M-C, Alliot-Licht B. Phenotypic analysis of immunocompetent cells in healthy human dental pulp. J Endod 2015 ;41(5):621-7. doi : 10.1016/j.joen.2015.01.005.
– Bonnamain V, Thinard R, Sergent-Tanguy S, Huet P, Bienvenu G, Naveilhan P, Farges J-C, Alliot-Licht B. Human dental pulp stem cells cultured in serum-free supplemented medium. Front Physiol 2013 ;4:357. doi:10.3389/fphys.2013.00357.
– Farges J-C, Alliot-Licht B, Baudouin C, Msika P, Bleicher F, Carrouel F. Odontoblast control of dental pulp inflammation triggered by cariogenic bacteria. Front Physiol 2013 ;4:326. doi : 10.3389/fphys.2013.00326.
Collaborations :
-Pr Colin McGUCKIN & Dr Nico FORRAZ, CTI-BIOTECH, Meyzieu.
-Dr Audrey AUSSEL, Dr Nicolas L’heureux, Dr Olivia Kérourédan, Laboratoire Biotiss, Bordeaux.



