Project leader : S. Vadon-Le Goff
BTPs and their partners (PCPEs, meprins α and β) represent potential therapeutic targets for a large number of pathologies (fibrosis, corneal scarring, bone fractures etc). In some cases, we would like to increase their activity but, in most cases, inhibition is required. In collaboration with a group at the CEA Saclay, we recently developed a new family of synthetic inhibitors of BTPs and meprins. We are presently comparing their properties (efficiency, toxicity, specificity) with previously described molecules.
We also seek inspiration from the biological properties of some natural proteins to design novel inhibitors. For example, we are currently elucidating the mechanism of action of the Xenopus protein called sizzled, a potent and highly specific inhibitor of human BTPs (Figure), in order to improve existing inhibitors. In addition, another major goal in the group is to develop antagonists of PCPE activity, both through rational design based on structural information and through screening of libraries.
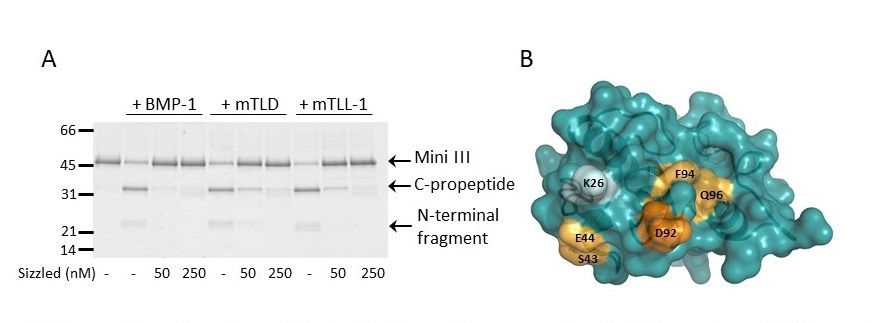
(A) The sizzled protein from Xenopus efficiently inhibits human BTPs (BMP-1, mTLD and mTLL-1), in the presence of a model substrate derived from procollagen III (Mini III). (B) Residues in the frizzled domain of sizzled which were identified as being involved in the interaction with BMP-1 by site-directed mutagenesis (blue : not involved; light orange : significantly involved; bright orange: strongly involved). Adapted from Bijakowski et al. J. Biol. Chem. 2012.
Mains techniques used
• Enzyme assays
• Surface Plasmon Resonance, ELISA
• Crystallography
• Library screening
• Cellular assays
Selection of publications :
Inhibitors of BMP-1/tolloid-like proteinases: efficacy, selectivity and cellular toxicity. Talantikite M, Lécorché P, Beau F, Damour O, Becker-Pauly C, Ho WB, Dive V, Vadon-Le Goff S, Moali C. FEBS Open Bio. 2018;8(12):2011-2021.
Sizzled is unique among secreted frizzled-related proteins for its ability to specifically inhibit bone morphogenetic protein-1 (BMP-1)/tolloid-like proteinases. Bijakowski C, Vadon-Le Goff S, Delolme F, Bourhis JM, Lécorché P, Ruggiero F, Becker-Pauly C, Yiallouros I, Stöcker W, Dive V, Hulmes DJ, Moali C. J Biol Chem. 2012;287(40):33581-93.



