Project 2A. miR-30 function in aging, effect on autophagy, transmission of aging message in kerat through exosomes. Project leader : F. Chevalier
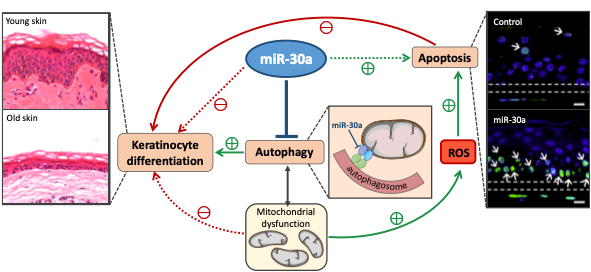
Epidermal homeostasis is maintained by genetics mechanisms which are progressively disturbed with chronological aging. We have recently identified several small non-coding RNAs (micro-RNAs) modulated in human epidermis with aging, especially miR-30a which overexpression disturbs the epidermal barrier function (Muther C. et al. 2017).
This project aims at deciphering the molecular mechanisms under the control of miR-30a. Our main hypothesis is that miR-30a is a key regulator of autophagy, which will be dysregulated with aging and thus will impact both keratinocyte differentiation and survival. In addition, we have observed miR-30a enrichment in the exosomes released by old keratinocytes. We are thus interested in miR-30a spreading across the epidermis through exosomes, and the fate of the recipient cells.
Recent publication of the group on the topic:
Muther C, Jobeili L, Garion M, Heraud S, Thepot A, Damour O, Lamartine J. An expression screen for aged-dependent microRNAs identifies miR-30a as a key regulator of aging features in human epidermis. Aging 2017 Nov 19 ;9(11) :2376-2396
Project 2B – Role of TRPV1 in psoriasis-associated vascular inflammation. Project leader : B. Fromy
Widely expressed in skin sensory nerve endings, transient receptor potential vanilloid receptor subtype 1 (TRPV1) is also expressed by epidermal keratinocytes and cutaneous microvessels. We thus hypothesize that, in addition to nervous TRPV1, the TRPV1 receptors on non-neuronal cells could be involved in the vascular inflammation associated with psoriasis.
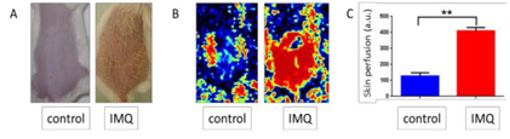
One of the most widely approved psoriasis-like animal models is the imiquimod (IMQ)-induced mouse model that we previously used in our lab (Jabeen et al. Pharmaceutics 2020, Boisgard et al. Eur J Pharm Biopharm 2017). Our preliminary data show that these psoriasis-like lesions were associated with vascular inflammation (as shown below).
This project aims 1) to identify the role of TRPV1 and which TRPV1 expressing cells play a key role in the inflammatory vicious circle, 2) to study the implication of microvasculature which is poorly studied in the context of psoriasis, 3) to understand the communications between different cellular types (neurons, basal and differentiated keratinocytes, and endothelial cells) in regards of neuropeptides, cytokines and TRPV1 activation.
Recent publications of the group on this topic
Jabeen M, Boisgard AS, Danoy A, El Kholti N, Salvi JP, Boulieu R, Fromy B, Verrier B, Lamrayah M. Advanced Characterization of Imiquimod-Induced Psoriasis-Like Mouse Model. Pharmaceutics. 2020; 12(9):789. doi: 10.3390/pharmaceutics12090789.
Boisgard AS, Lamrayah M, Dzikowski M, Salmon D, Kirilov P, Primard C, Pirot F, Fromy B, Verrier B. Innovative drug vehicle for local treatment of inflammatory skin diseases: ex vivo and in vivo screening of five topical formulations containing poly(lactic acid) (PLA) nanoparticles. Eur J Pharm Biopharm 2017; 116: 51-60.
Project 2C – Role of Na+ and Cl- in vascular dysfunction. Project leader : N. Picard
Body sodium (Na+) and chloride composition are tightly regulated variables influencing numerous physiological functions such as extracellular volume (composition and volume of liquid between cells). Many studies in humans and mammalians showed that excessive dietary NaCl (salt) intake is a risk factor worsening adverse consequences of hypertension or diabetes. Salt restriction is often provided as a first-line dietary and preventive recommendations to limit the disease-associated insults on blood vessels, heart, and kidneys. Salt balance is tightly regulated between the dietary entries of NaCl and the urinary output generated by the kidneys. The skin (and muscles) play an important role in salt balance regulation recently highlighted by several studies.
Our project has several aims:
– Understanding the mechanisms regulating skin sodium chloride content
– Determine the influence of these ions on vessels plasticity and functionality (vasodilation and vasoconstriction) and understand their role in vascular dysfunction
– Describe the renal mechanisms regulating body and skin NaCl content. We focus on a specific cell type in the kidney: the intercalated cells also called mitochondria rich cells which are known to be implicated in sodium, chloride, and protons balance