Project leader: Claire Monge (CR CNRS)
People involved: Evelyne Colomb (IR), Anne-Lise Paris (PhD student ANRS), Mehwish Jabeen (PhD student), Sophie Richard (MCU), Danielle Arruda (MCU), Renaud Rovera (T), Céline Coiffier (IE).
Grants: ANR JCJC BuccaVac, Sidaction, ANRS, SATT Pulsalys
Collaborations: Fabienne Anjuère (IPMC, Sophia-Antipolis), R Wagner (European HIV Vaccine Alliance), N. Dereuddre-Bosquet et Roger LeGrand ( CEA Fontenay aux Roses ), Stéphane Paul (GIMAP St Etienne), R. Cox (Flu Vaccine institute, Bergen, Norway), J. Tregonning (Imperial College London), PLATIM, ANICAN, PBES
One of our main concern is to use materials and reagents that are eco-friendly. The only organic solvants that are used are acetone and DMSO, considered are environment friendly. No toxic coupling agent is used, and we tend to full green chemistry processes.
Induction of mucosal immunity by bioinspired delivery systems.
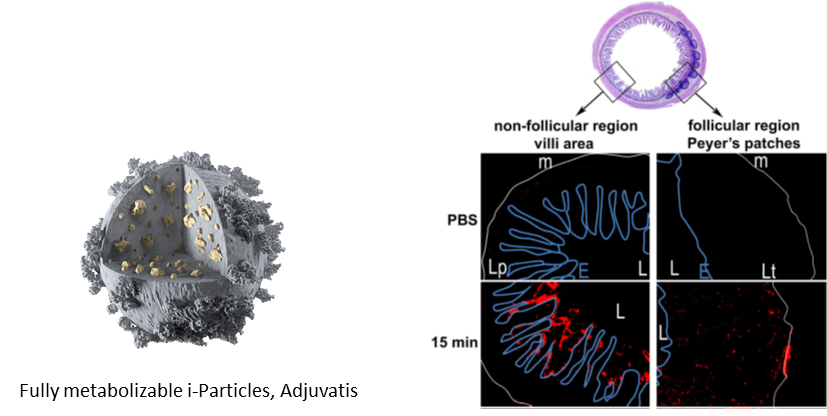
Mucosal immune system is functionally independent of the systemic immune system, and has developed its own highly organized lymphoid tissues to maintain tissue homeostasis (anti-inflammatory cytokines, tolerance) or rather induce a strong mucosal immune response (in particular secretory IgA). Mucosal immunity is currently at the centre of intense vaccine research since the discovery of its potential in fighting infections. Indeed, it is the first line of defence by inducing a strong local mucosal response together with a systemic response. Our previous works on nasal and intestinal delivery of nanoparticles led to gain knowledge about the mechanisms involved in the mucosal response to a nanoparticulate vaccine antigen, ie adsorbed onto a PLA-nanoparticles.
Fluorescent micrographs obtained from transversal cryosections of mice ileum intestine. In vivo intestinal ligated loop was incubated with10mg/mlofCellTraceBODIPY fluoNPs diluted in PBS for 15min.
Two strategies are currently developed to induce mucosal immunity using bioinspired delivery systems:
1/ direct mucosal administration through buccal delivery of antigen
2/ induction of a mucosal immunity by the delivery of nanoparticles carrying a mucosal adjuvant.
Sublingual vaccination: evaluation of the immune response to buccal administration of vaccines by LbL patches.
Since the early 2000’s, increasing attention has been dedicated to the buccal mucosa as an alternative route for drug administration because of its excellent accessibility and physical robustness. Besides, this administration route allows the avoidance of intestinal and hepatic metabolism, and presents low enzymatic activity. The buccal mucosa being a more permeable membrane than skin, it is particularly favourable for protein or macromolecule delivery. For vaccine development, the sublingual route is known to present a large dissemination of immune responses at distant sites and provide both systemic and mucosal antibody response (Paris et al, Journal of Controlled Release, 2021).
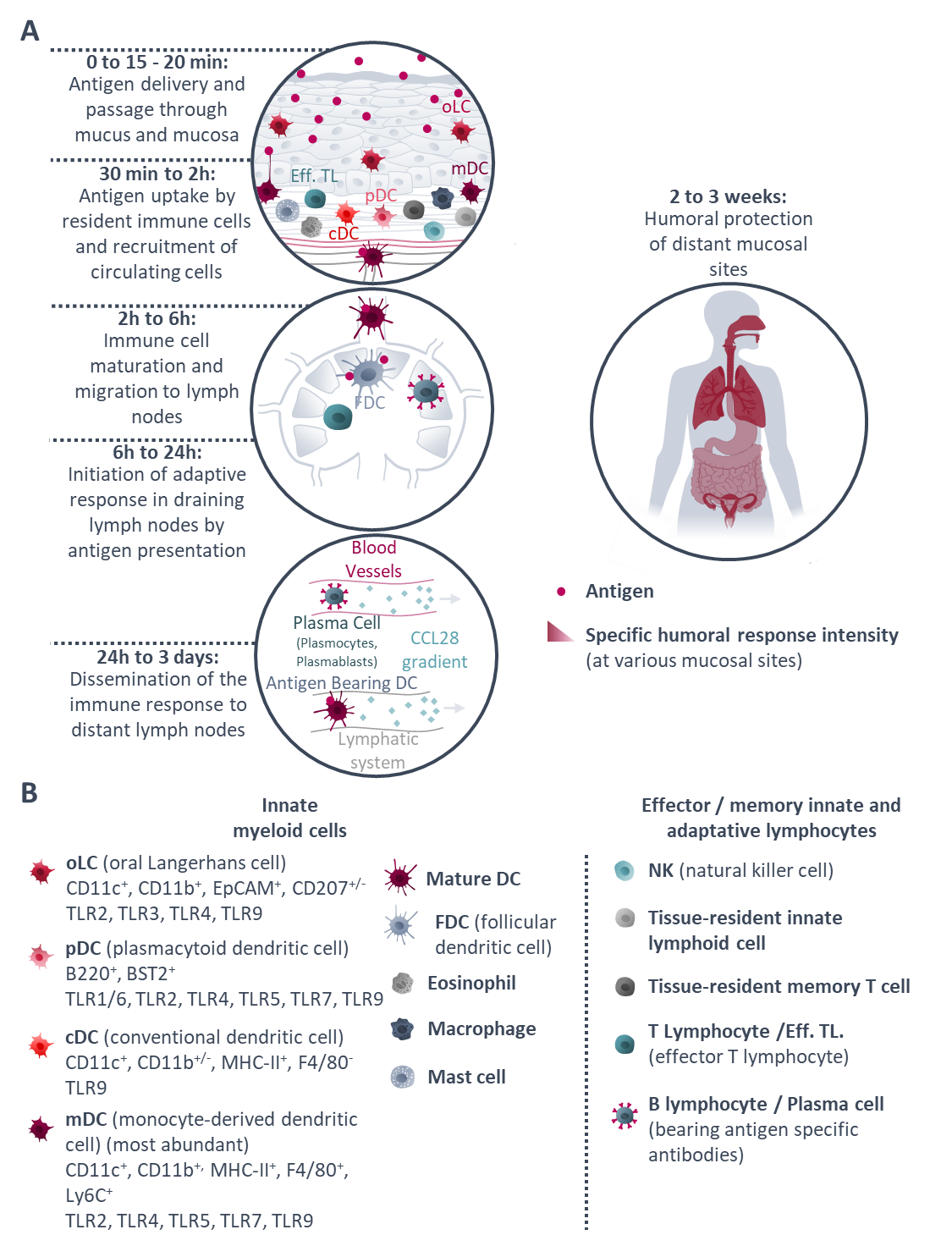
Insight on the main phases of the immune response after sublingual vaccination: from antigen delivery to distant mucosal site protection. From Paris et al, 2021, Journal of Controlled Release.
By the development of a controlled delivery system for sublingual vaccine our ambition is to maximize vaccine efficacy and develop the buccal route as a novel administration way.
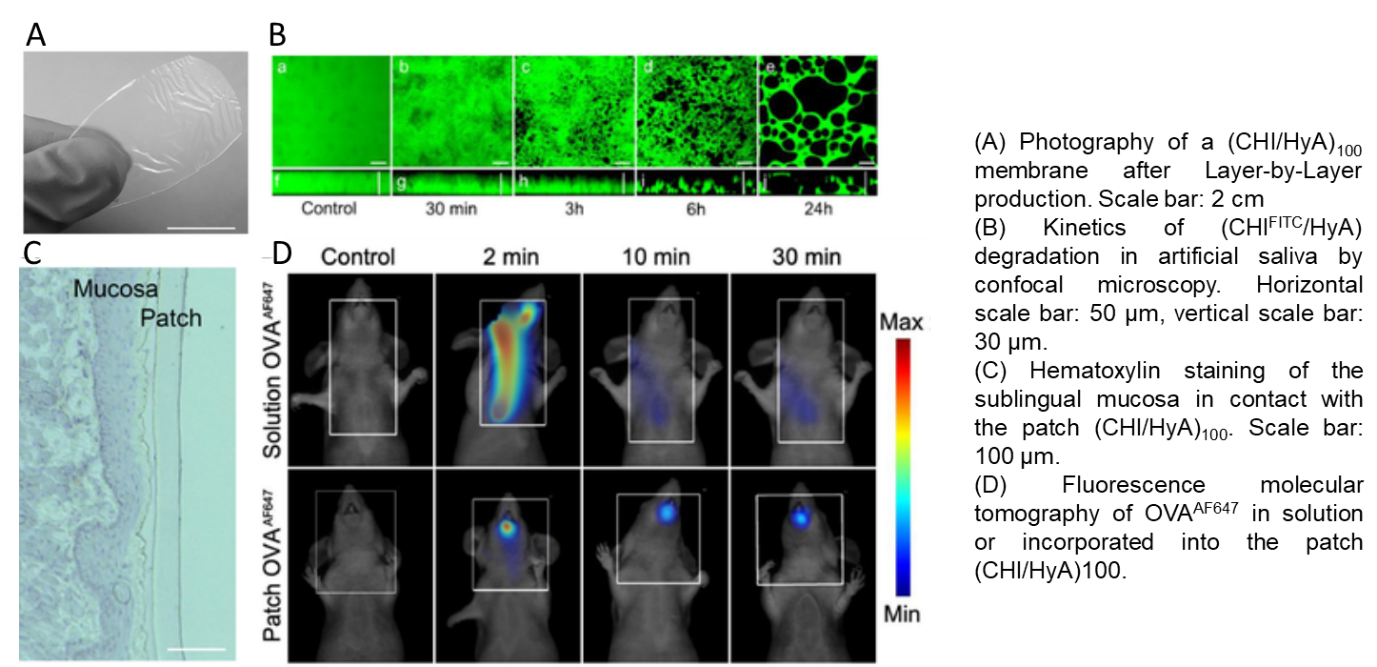
Thus, our project relies on the development of transmucosal delivery systems by the conception of orodispersible patches to deliver vaccine formulation or other simpler biomacromolecules (proteins). The patch is produced by the Layer-by-Layer technology (Monge et al, Acta Biomaterialia, 2015; and for review see Monge et al, Advanced Healthcare Materials, 2015) and is composed by optimized natural polymers (polysaccharides) of opposite charges (chitosan/hyaluronic acid), it is fully biocompatible, mucoadhesive and biodegradable in saliva. The presence of the patch in the oral cavity increases the contact time between the macromolecule and the mucosa facilitating its diffusion (Paris et al, Acta Biomaterialia, 2021).
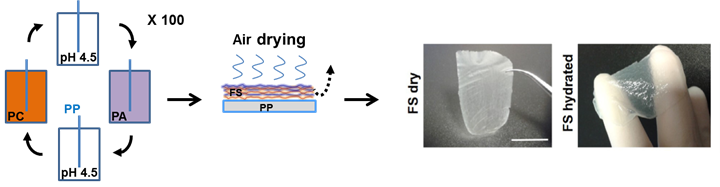 Freestanding membrane made of Layer-by-Layer assembly of natural polycations and polyanions.
Freestanding membrane made of Layer-by-Layer assembly of natural polycations and polyanions.
Natural polymers: Seaweed polysaccharides against respiratory viral infections
Respiratory viral infections have been a leading cause of morbidity and mortality world-wide. Despite massive advancements in the virology field, no specific treatment exists for the majority of respiratory viral infections. So far, approved therapies against respiratory viruses almost exclusively rely on synthetic drugs that have potential side effects therefore restricting their use. This review aims at presenting natural marine sulfated polysaccharides possessing promising anti-viral activity against respiratory viruses that could be a safe alternative to synthetic broad-spectrum antiviral drugs. In the review Jabeen et al, we present antiviral properties of marine sulfated polysaccharides according to their mechanism of action on different types and strains of respiratory viruses.
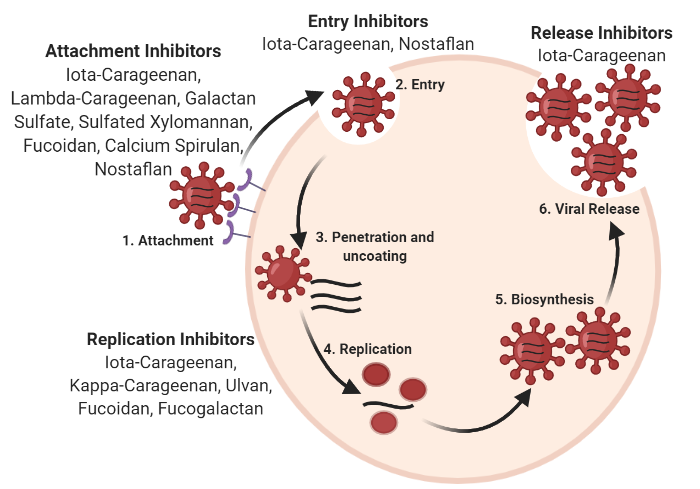
Mechanism of action of marine sulfated polysaccharides at various stages of viral cycle. From Jabeen et al, Pharmaceutics, 2021
Selection of publications :
Paris AL, Colomb E, Verrier B, Anjuère F, Monge C. (2021) Sublingual vaccination and delivery systems. Journal of Controlled Release. 332:553-562.
Monge C, Verrier B. (2021) Sublingual antigen delivery: a solution for needle-free HIV vaccination. Expert Review of Vaccines. Editorial,1-4.
Paris AL, Caridade S, Colomb E, Bellina M, Boucard E, Verrier B, Monge C. (2021) Sublingual protein delivery by a mucoadhesive patch made of natural polymers. Acta Biomaterialia. 128:222-235.
Jabeen M, Dutot M, Fagon R, Verrier B, Monge C. (2021) Seaweed Sulfated Polysaccharides against Respiratory Viral Infections. Pharmaceutics.13(5):733.
Coolen AL, Lacroix C, Mercier-Gouy P, Delaune E, Monge C, Exposito JY, Verrier B. (2019) Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials. 2019;195:23-37.
Berthet M, Gauthier Y, Verrier B*, Monge C* (2018) Nanoparticle-Based Dressing: The Future of Wound Treatment? Trends in Biotechnology. 36(1):119.
Monge C, Almodóvar J, Boudou T, Picart C. (2015) Spatio-Temporal Control of LbL Films for Biomedical Applications: From 2D to 3D. Adv Healthc Mater. 4(6):811-30.
Monge C*, Caridade SG*, Almodóvar J, Guillot R, Lavaud J, Josserand V, Coll JL, Mano JF, Picart C. (2015) Myoconductive and osteoinductive free-standing polysaccharide membranes. Acta Biomater. 15:139-49.
Caridade SG, Monge C, Gilde F, Boudou T, Mano JF, Picart C. (2013) Free-standing polyelectrolyte membranes made of chitosan and alginate. Biomacromolecules. 2013 May 13;14(5):1653-60.