Principal investigator: Romain Debret (CR)
Research group members: Aurore Berthier (IA), Sarah Chanteloube (Doc.), Chloé Chave (Doc.), Marie Hoareau (Doc.)
Collaborators at LBTI: Ming Lo (MCF, Research Group 1), Kiaoling Liu Lo (MCF, RG 1), Elise Lambert (MCF, RG 3), Jérôme Sohier (CR, RG 5), Danielle Campiol-Arruda (MCF, RG 5), Fabrice Pirot (PU-PH, RG 5), Jean-Paul Salvi (TCN, RG 5)
Academic collaborators: Serge Adnot (IMRB, Créteil), Stéphane Avril (Sainbiose, Saint-Etienne), Gilles Faury (HP2, Grenoble), Daniella Quaglino (Université de Modène, Italie), Dieter Reinhardt (Université McGill, Canada), Cyril Pailler-Mattéi (LTDS, Ecully)
Industrial partnership: Lucas Meyer Cosmetics, Isispharma, BASF-Beauty Care Solutions
Public funding: ANR « Arterylastic » (www.arterylastic.com), ANR « TherAlveo »
The physiological functions of many tissues rely on their mechanical elastic properties. Elastic fibers are essential components of the extracellular matrix (ECM), responsible for the resilience of vertebrate tissues. These structures are found in abundance in tissues such as skin, arteries, lungs and ligaments, which are subjected to high mechanical stresses requiring repeated cycles of extension and return to their original shape. These fibers are made up of 90% elastin, a polymer formed by the cross-linking of tropoelastin, and 10% fibrillin-rich microfibrils. The maturation of the whole is ensured by the enzymatic action of the lysyl oxidase family. Elastic fibers are synthesized (elastogenesis) during the last third of the fetal period, and a peri-natal synthesis peak is observed before gradually decreasing during growth. Upon reaching adulthood (about 20 years in humans), elastogenesis is very weak and the renewal of the fibers is only partial or non-functional. Thus, the elastic capital built up at the young age gradually decreases over time with significant consequences on physiological functions. Aging is therefore linked to the integrity of the elastic fibers. As a result, genetic syndromic pathologies (cutis laxa, Williams-Beuren) which affect the elastic fibers, or any pathological situation causing an exacerbate destruction of the fibers (inflammation, malnutrition, stress, etc.), participate in the establishment of a localized or systemic progeroid phenotype. The ACTE research group aims to understand the impact of changes in elasticity on cell behavior, and aims to develop innovative therapies to restore tissue elasticity with the skin as a study model. Our projects are therefore organized along two distinct axes.
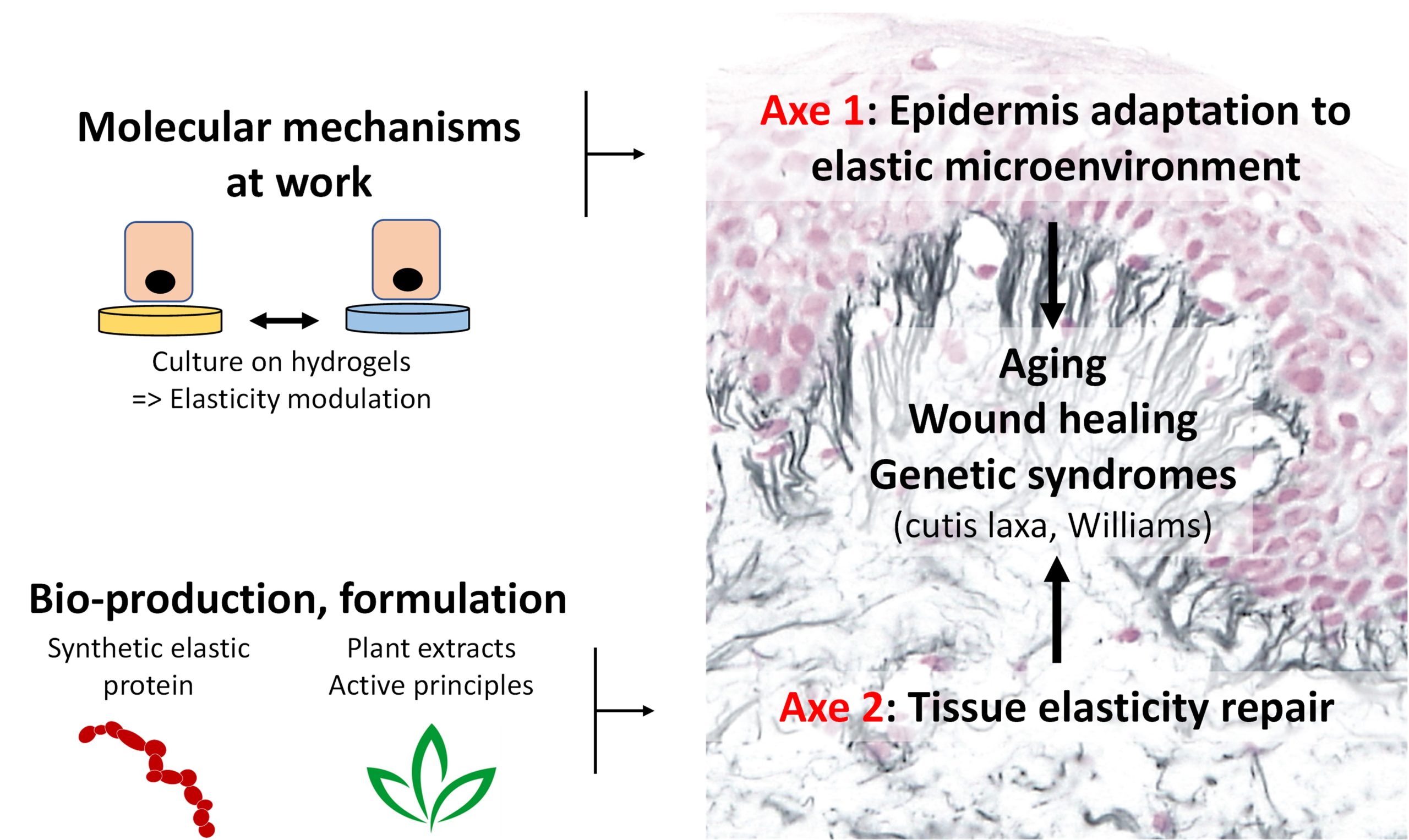
ACTE overall strategy
AXE I: Epidermis adaptation to elastic microenvironment
This axis with strong mechanobiological connotation aims at determining the molecular mechanisms allowing keratinocytes (epidermal main cells) to respond to changes in rigidity/elasticity in the contexts of skin aging and wound healing for which keratinocytes are exposed to strong variations in their mechanical environment.
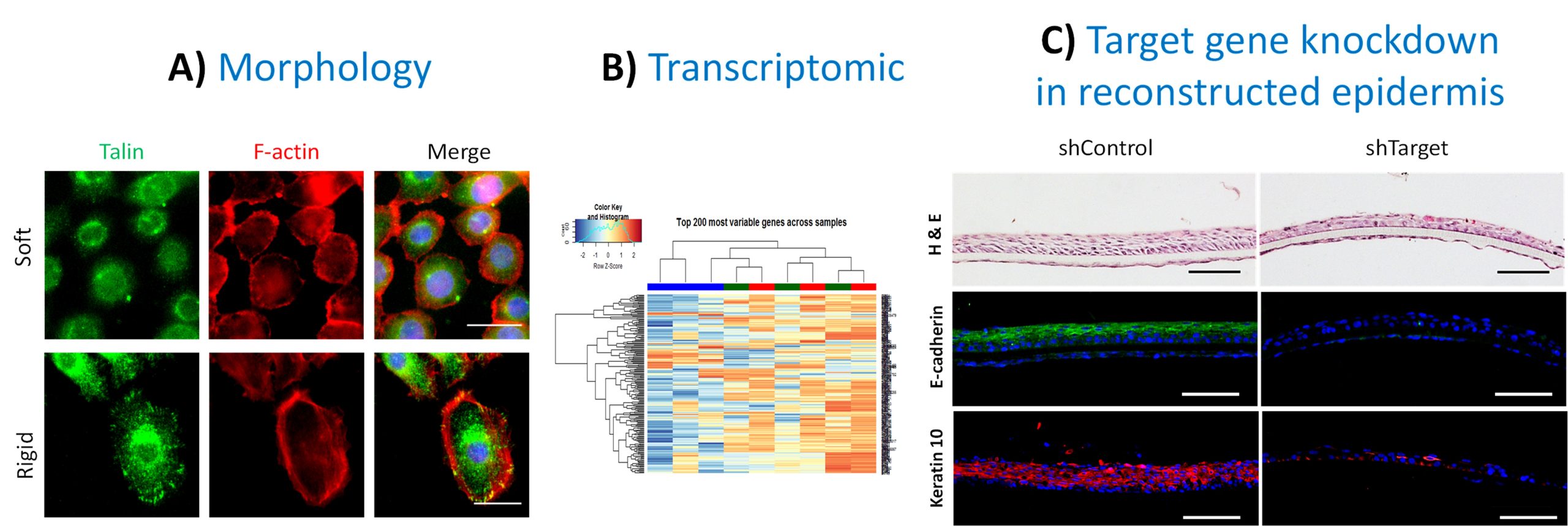
Identification of new mechano-receptive pathways in the keratinocyte
A) Analyzes of keratinocyte morphology in response to stiffness by fluorescence microscopy. Scale bar = 25 µm. B) Analysis of the keratinocyte transcriptome by RNA sequencing and identification of mechano-responsive targets. C) Reconstruction of epidermis from invalidated keratinocytes for a target identified in RNAseq. Scale bar = 100 µm.
AXE II: Tissue elasticity repair
This ambitious axis intends for the first time to provide pharmacological solutions to elasticity defects at tissue level (skin, vessels, lungs), or even at whole organism level since elasticity is systemic. For this, 2 complementary strategies are developed: 1) the purification of active plant extracts; 2) the use of a synthetic elastic protein (SEP) as a bio-therapeutic agent.
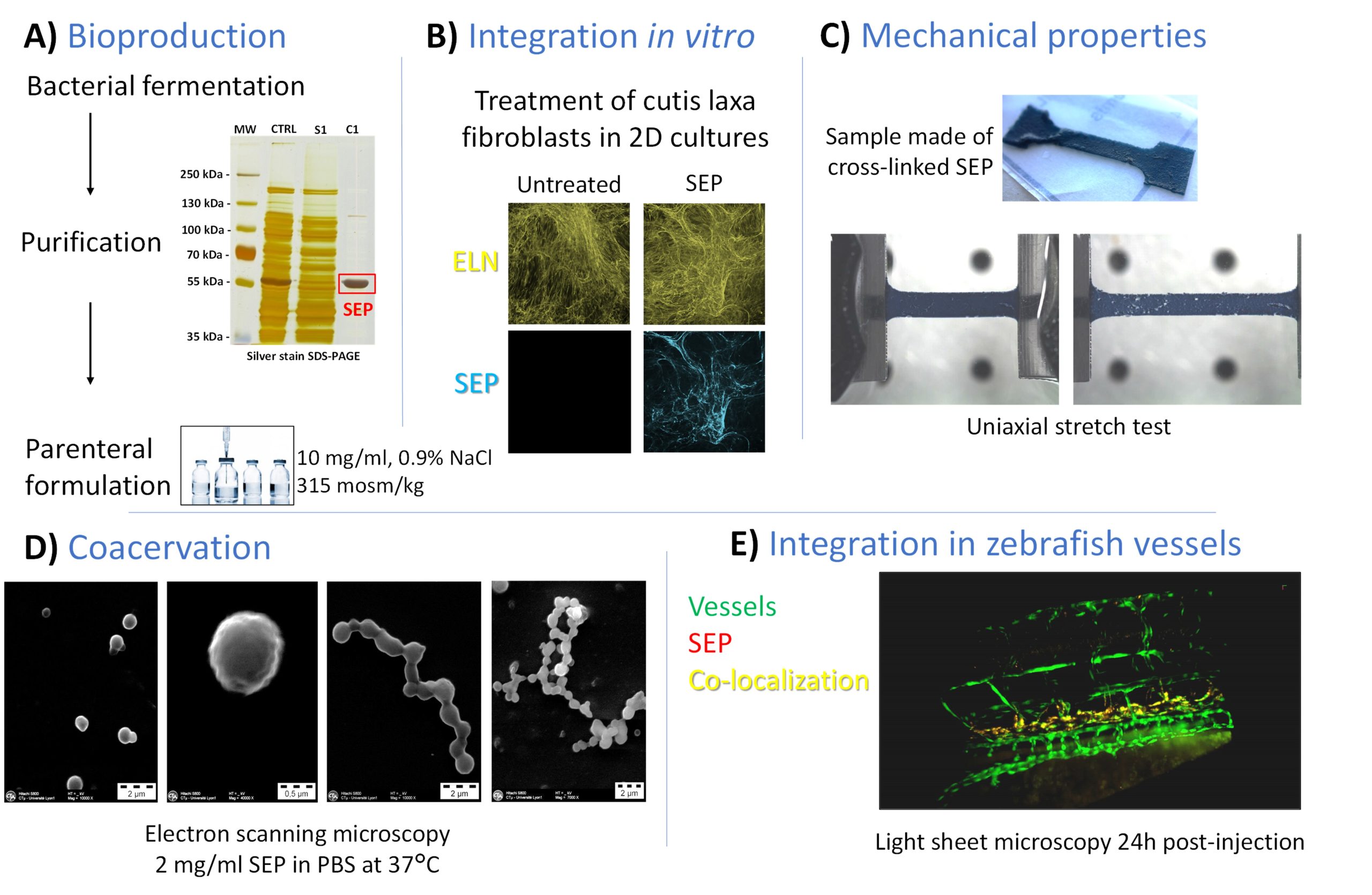
Synthetic elastic protein (SEP) production and characterization
A) Bioproduction in a prokaryotic system coupled with purification by Inverse Transition Cycling and data from the parenteral formulation. B) Example of SEP integration in tropoelastin fibrillar deposits from cultured cutis laxa cells. C) Illustration of the purely elastic behavior of the SEP during uniaxial tensile test measurements. D) Coacervation at 37°C similar to native tropoelastin. E) Light sheet microscopy 24h post-injection in zebrafish.
Representative articles:
Hoareau M, El Kholti N, Debret R, Lambert E. Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies. Int J Mol Sci. 2022 Feb 14;23(4):2102. doi: 10.3390/ijms23042102.
Boizot J, Minville-Walz M, Reinhardt DP, Bouschbacher M, Sommer P, Sigaudo-Roussel D, Debret R. FBN2 Silencing Recapitulates Hypoxic Conditions and Induces Elastic Fiber Impairment in Human Dermal Fibroblasts. Int J Mol Sci. 2022 Feb 5;23(3):1824. doi: 10.3390/ijms23031824.
Ya C, Carrancá M, Sigaudo-Roussel D, Faure P, Fromy B, Debret R. Substrate softness promotes terminal differentiation of human keratinocytes without altering their ability to proliferate back into a rigid environment. Arch Dermatol Res. 2019 Dec;311(10):741-751. doi: 10.1007/s00403-019-01962-5.
Mainzer C, Remoué N, Molinari J, Rousselle P, Barricchello C, Lago JC, Sommer P, Sigaudo-Roussel D, Debret R. In vitro epidermis model mimicking IGF-1-specific age-related decline. Exp Dermatol. 2018 May;27(5):537-543. doi: 10.1111/exd.13547.
Moulin L, Cenizo V, Antu AN, André V, Pain S, Sommer P, Debret R. Methylation of LOXL1 Promoter by DNMT3A in Aged Human Skin Fibroblasts. Rejuvenation Res. 2017 Apr;20(2):103-110. doi: 10.1089/rej.2016.1832.
Boraldi F, Bartolomeo A, Annovi G, Debret R, Quaglino D. Magnesium Modifies the Structural Features of Enzymatically Mineralized Collagen Gels Affecting the Retraction Capabilities of Human Dermal Fibroblasts Embedded within This 3D System. Materials (Basel). 2016 Jun 15;9(6):477. doi: 10.3390/ma9060477.
Debret R, Cenizo V, Aimond G, André V, Devillers M, Rouvet I, Mégarbané A, Damour O, Sommer P. Epigenetic silencing of lysyl oxidase-like-1 through DNA hypermethylation in an autosomal recessive cutis laxa case. J Invest Dermatol. 2010 Nov;130(11):2594-601. doi: 10.1038/jid.2010.186.



