Porteur de projet : Jean-Daniel Malcor
Personnes impliquées dans le projet : Marielle Pasdeloup, Frédéric Mallein-Gerin, Jérôme Lafont
Cartilage is an avascular tissue which possesses poor intrinsic healing properties. As osteoarthritis is a growing source of infirmity worldwide, cartilage repair represents an increasingly important clinical challenge. Our goal is to develop biomimetic approaches for cartilage tissue engineering. We design and fabricate biomaterials to host chondrocytes, the cellular component of cartilage, or mesenchymal stem cells (MSCs) derived from adipose tissue or dental pulp, due to their high proliferative abilities and versatile differentiation potential.
Our biomaterials also provide cells with the correct biological and mechanical cues to direct cellular response, including cell adhesion, survival, differentiation and function. In cartilage, the extracellular matrix (ECM), and in particular collagen, plays a predominant role in providing a biological and structural support for chondrocytes. We aim to replicate the key characteristics of the ECM in biomaterials seeded with chondrocytes or MSCs, and to produce a cellular microenvironment suitable with the formation of artificial engineered cartilage.
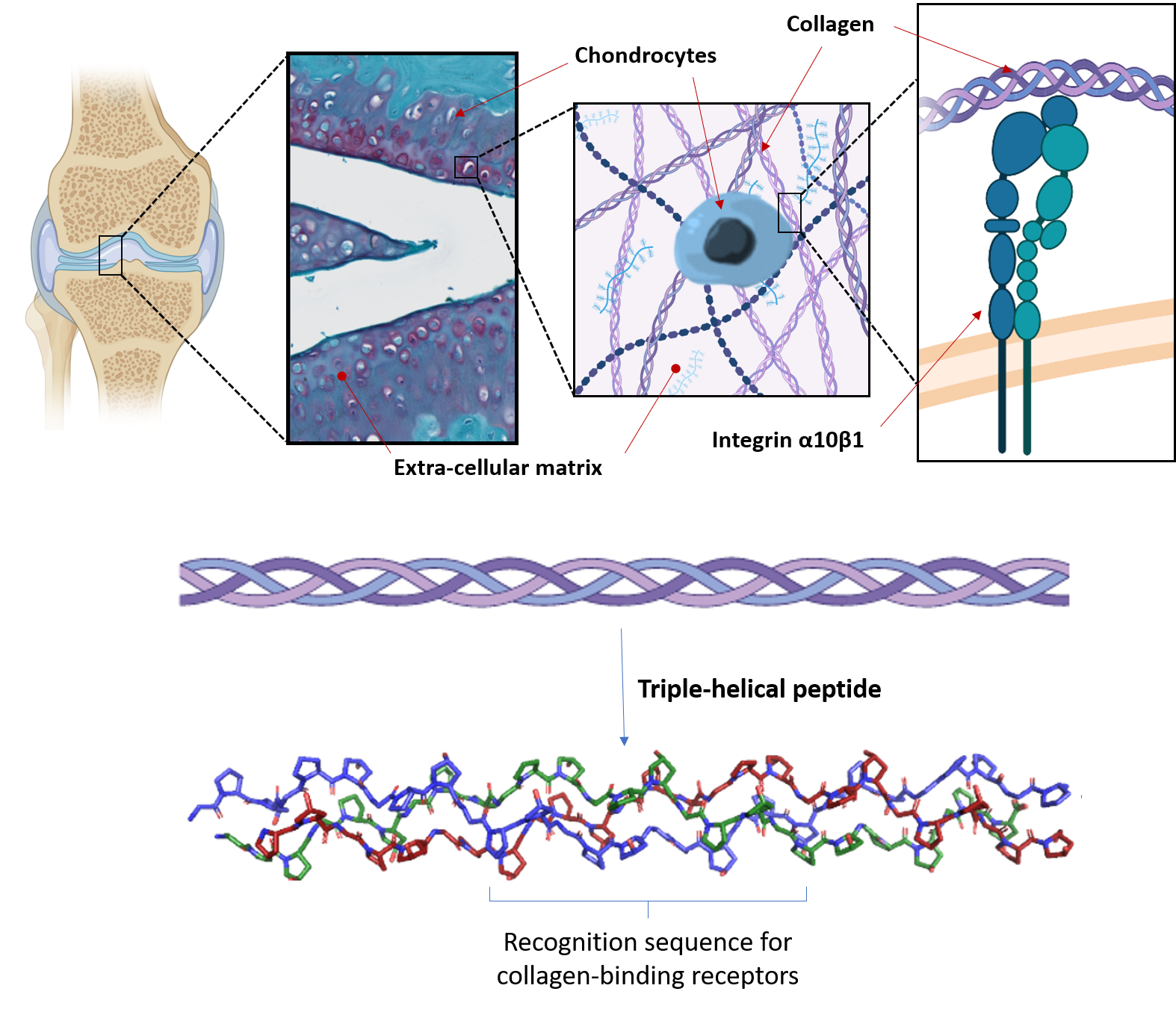
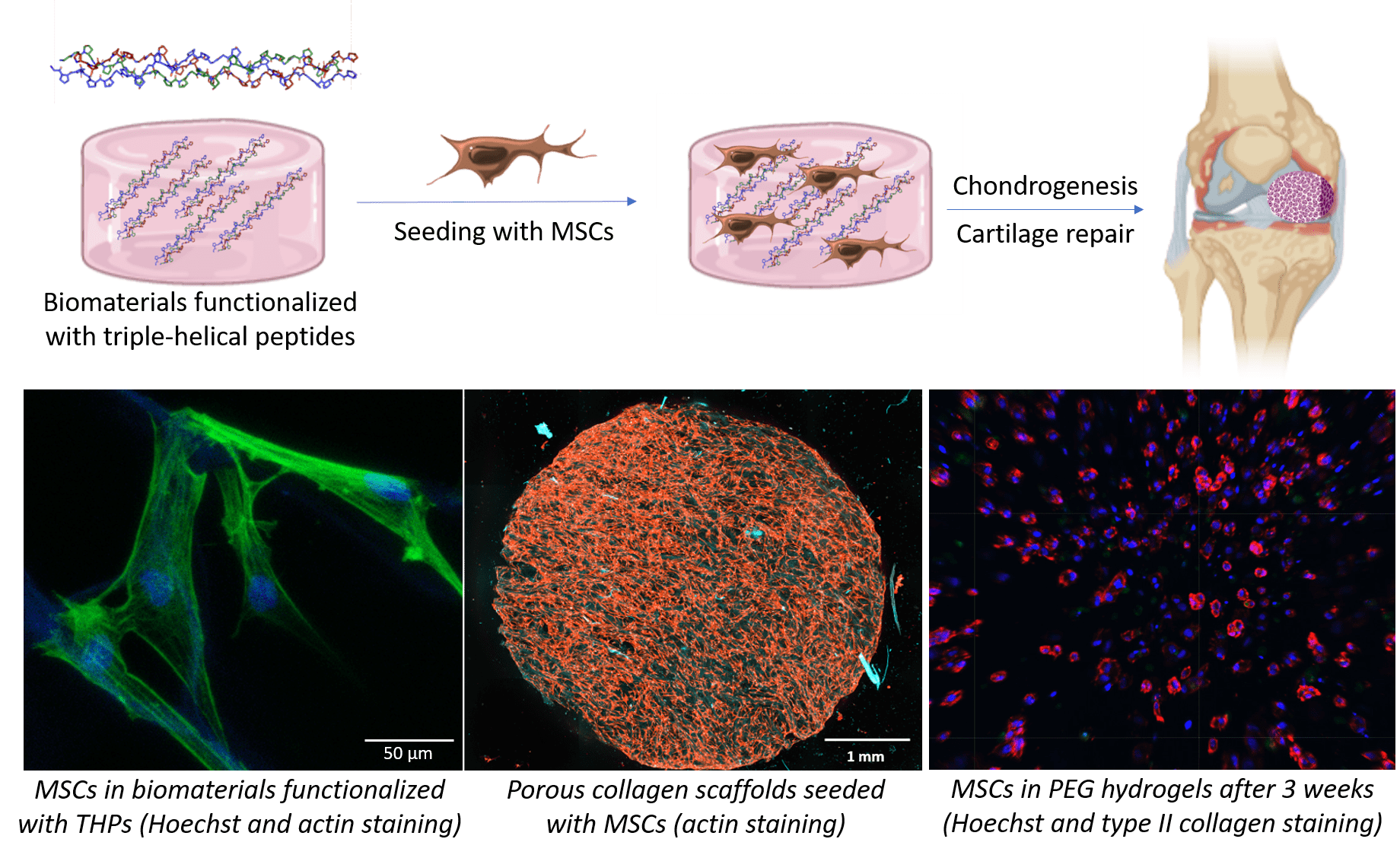
Our strategy is to employ triple-helical peptides (THPs), a family of biomimetic peptides that spontaneously assemble into a triple helix conformation that mimic the structure of collagen. We use THPs containing specific sequences that are ligands for collagen-binding receptors to replicate cellular interactions with collagen. Targeting key cell-surface receptors (such as the collagen-binding integrins α1β1, α2β1, α10β1 and α11β1, or the discoidin domain receptors 1 and 2) enables us to understand how collagen recognition precisely affect MSC fate and chondrocyte activity. This fundamental insight allows us to drive chondrogenesis and cartilage ECM production in biomaterials made from collagen, fibrin, PEG or agarose, both as hydrogels or porous scaffolds. In addition, we have developed crosslinking methodologies to improve the stability and rigidity of these biomaterials, making them suitable with the mechanical loads found in articular cartilage without affecting their biological activity.
We also employ THPs to specifically target the α10β1 integrin, a chondrocyte marker that plays a central role in cartilage homeostasis, to selectively retain chondrogenic MSCs and prompt their differentiation into chondrocytes. THP-functionalized cell-laden biomaterials will constitute, on the one hand, a platform to model cartilage in vitro and to conveniently study pathologies associated with cartilage (such as osteoarthritis) at a reduced cost; and on the other hand, patches destined to be implanted on damaged cartilage in vivo, to contribute to the repair of articular cartilage following traumatic injury.
Selected publications
1. A. Ziverec, D. Bax, R. Cameron, S. Best, M. Pasdeloup, E-J. Courtial, F. Mallein-Gerin, J-D. Malcor, The diazirine-mediated photo-crosslinking of collagen improves biomaterial mechanical properties and cellular interactions, Acta Biomater. (2024).
2. J-D. Malcor and F. Mallein-Gerin, Biomaterial functionalization with triple-helical peptides for tissue engineering, Acta Biomater.148 (2022) 1-21.
3. J-D. Malcor, V. Juskaite, D. Gavriilidou, E.J. Hunter, N. Davidenko, S. Hamaia, S. Sinha, R.E. Cameron, S.M. Best, B. Leitinger, R.W. Farndale, Coupling of a specific photoreactive triple-helical peptide to crosslinked collagen films restores binding and activation of DDR2 and VWF, Biomaterials 182 (2018) 21-34.
4. J-D. Malcor, D. Bax, S.W. Hamaia, N. Davidenko, S. Best, R. Cameron, R. Farndale, D. Bihan, The synthesis and coupling of photoreactive collagen-based peptides to restore integrin reactivity to an inert substrate, chemically-crosslinked collagen, Biomaterials 85 (2016) 65-77.
5. J-D. Malcor, E. Hunter, N. Davidenko, B. Bax, S. Best, R. Cameron, S. Sinha, R. Farndale, Collagen scaffolds functionalized with triple-helical peptides support 3D HUVEC culture, Regenerative Biomaterials 5 (2020) 7 471-481.
6. M. Colzani, J.D. Malcor, E.J. Hunter, S. Bayraktar, M. Polkinghorne, R. Cameron, S. Best, S. Sinha, R. Farndale, Modulating hESC-derived cardiomyocyte and endothelial cell function with triple-helical peptides for heart tissue engineering, Biomaterials 269 (2021) 120612.



