Project leader : Bernard Verrier (DR)
Participants : JY Exposito (CR CNRS), Claire Monge (CR CNRS), Sergio Linares (PhD student), Danielle Arruda (MCU), Pierre Libeau (AI), Céline Terrat (AI).
Our research group is involved is vaccine design since more than 20 years, first for designing HIV vaccine, which still remain a biggest challenge. During this quest, we have developed a new vaccine concept based on PLA nanoparticles, with an exciting observation in the last couple of years. Indeed, it appears that we could induce strong mucosal immunity by parenteral routes instead of mucosal routes, if we load particles with specific immune molecules and/or played around with the routes of skin immunization (Dermal, transdermal, sub cutaneous). As most pathogens enter through mucosal routes, designing vaccine formulation able to induce mucosal immunity after skin application is off importance and we will pursue this aim in three directions, with the help of several European network and lab forces.
Objective 1: Nanoparticles and innate immunity : Use of PRR ligands and nf kappa B signaling for intracellular manipulation of antigen presenting cells (HIV and staph Aureus model)
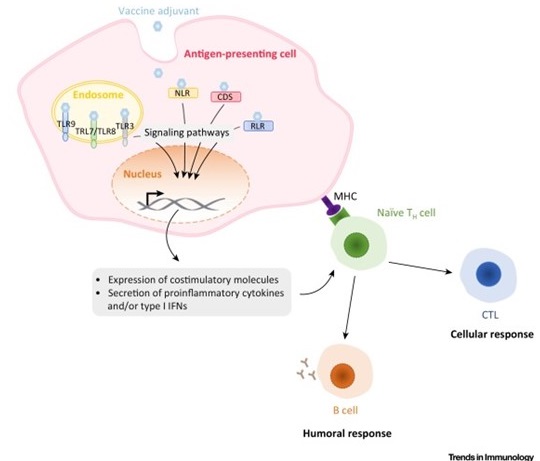
Mechanisms of Action of Adjuvants Targeting Intracellular Receptors. Intracellular pattern-recognition receptor (PRR) ligands stimulate antigen-presenting cells to express costimulatory molecules as well a proinflammatory cytokines and/or type I interferons (IFNs). This induces the recruitment of immune cells at the site of infection and directs the immune response towards type 1 or type 2 immunity. Abbreviations: CDS, cytosolic DNA sensor; CTL, cytotoxic T lymphocyte; MHC, major histocompatibility complex; NLR, NOD-like receptor; RLR, RIG-like receptor, TH cell, helper T cell; TLR, Toll-like receptor.
D’après Gutjahr et al, Trends Immunol. 2016 Sep;37(9):573-87
This task fits into the context of current vaccine research using innate immunity pathway for increasing adjuvant potency and optimization of sub-unit vaccine components. In fact, recognition of Pathogen-associated molecular patterns (PAMPS), through specific TLR (Toll Like receptors) or NLR (NOD-Like receptor) and identification of synthetic ligands, has opened a new area in adjuvant research. As such molecules could be encapsulated in biodegradable nanoparticles (NP), we could co-deliver to the same Antigen Presenting Cell (APC), both a vaccine antigen (such as HIV p24, or gp140 (Pierre Berge grant), or Staph Aureus antigen (BioAster grant) and the immune stimulatory molecules. Our working hypothesis is based on the fact that we could specifically target the cascade of regulation controlling PRR sensing through specific ligands (such as TLR2, NOD2, TLR7/8, or chimeric molecules (Pavot et al., Cutting edge, J. Immunity, in Press) and amplify or modulate immune responses directed against the antigen carried by the nanoparticle. In addition, the solid structure of these NPs made of Poly(Lactic Acid) promotes effective uptake and antigen processing by dendritic cells (DCs) after subcutaneous administration. Thus, the presence of immunostimulatory molecules in the core of NPs will guide the humoral and cellular response to the mucosal compartment and induce humoral broad spectrum and cell-mediated immune responses. To get insights on the mechanistic pathway involved in our previous observations, and the implication of specific cell subsets involved in the uptake of particles and their modulation, we will use transgenic mice model MYD 88(-/-) and TRIF (-/-), (PhD thesis of A. Gutjahr and C. Phelip) to identify immune readouts involved in mucosal immunity. Furthermore, our network of immunologists (S. Paul, B. Combadière, T. De France) will help us to dissect the fate of PRR adjuvanted particles upon skin administration (A.S. Boisgard) and in collaboration with other groups of LBTI. (O.Damour, (B. Fromy). It is of importance to notice that we have been associated as a partner in a ANR grant, starting in october 2016. (see MemoSign Abstract).
Objective 2 : PLA nanoparticles and sub unit vaccines
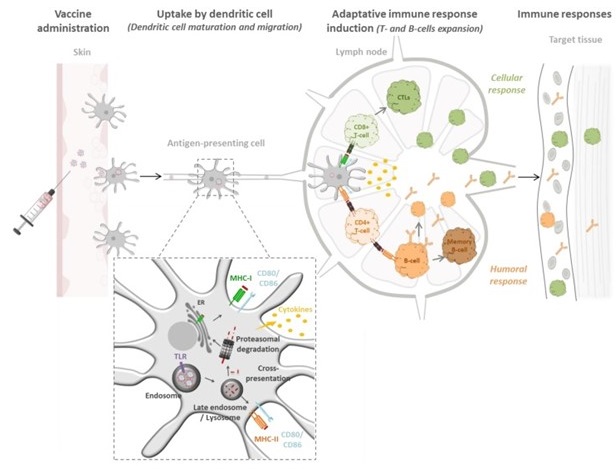
Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting
D’apres: Gutjahr A, Vaccines (Basel). 2016 Oct 12;4(4)
Objective 3 : PLA nanoparticles and mRNA vaccine
If PLA nanoparticles are very well suited for delivering vaccine antigen at their surface and encapsulated active agents in the inner core, their negatively charged surface charges preclude their direct use as Nucleic Acids carrier, such as mRNA or DNA in a vaccine context. Therefore, to circumvent this challenge and as an alternative to current approach based on encapsulation of nucleic acid in the core of PLA or PLGA carrier, we have taken advantage of a versatile tool, namely a Poly-Lysine polymer made of Dendri-Graft Poly-L-Lysine (DGL) and the expertise of the company Colcom (www.colcom.eu). Indeed, by creating a natural poly-lysine layer at the surface of the PLA particles (180nm), we have been able to adsorb different ratio of siRNA, different size of mRNA or DNA (from 5 to 19kb) after passive adsorption onto the Poly-Lysine corona loaded around the pre- formed PLA particles. In each case, we have been able to validate each assembly in relevant biological models as a proof of concept. For instance, we have reached high efficiency of DNA transfection after 6 days, using egfp-DNA without cell toxicity, siRNA delivery in a cancer model and mRNA in zebra fish model. These encouraging results prompt us to initiate new collaborations for further development of this platform as it exits room for improvement, especially for RNA vaccine. For instance, we expected that DGL layer will protect RNA from nuclease, and could favor cytosol release. Therefore, we will use an iterative approach, using different DGL formula or other natural polymers to identify the best formulation for mRNA delivery in a vaccine perspective. To explore such research area, we obtain two grants one in 2015 concerning HIV therapeutic vaccine (see abstract HIVERA : NanoVac) and one ANR grant Fish-RNAvax, coordinated by my group. (See abstract).
Selected publications :
Gutjahr A, Papagno L, Nicoli F, Lamoureux A, Vernejoul F, Lioux T, Gostick E, Price DA, Tiraby G, Perouzel E, Appay V, Verrier B, Paul S. (2017) Cutting Edge: A Dual TLR2 and TLR7 Ligand Induces Highly Potent Humoral and Cell-Mediated Immune Responses. J Immunol. 198(11):4205-4209.
Verrier B, Paul S, Terrat C, Bastide L, Ensinas A, Phelip C, Chanut B, Bulens-Grassigny L, Jospin F, Guillon C. (2017) Exploiting Natural Cross-reactivity between Human Immunodeficiency Virus (HIV)-1 p17 Protein and Anti-gp41 2F5 Antibody to Induce HIV-1 Neutralizing Responses In Vivo. Front Immunol. 8:770.
Gutjahr A, Phelip C, Coolen AL, Monge C, Boisgard AS, Paul S, Verrier B. (2016) Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines (Basel). 4(4).
Pavot V, Climent N, Rochereau N, Garcia F, Genin C, Tiraby G, Vernejoul F, Perouzel E, Lioux T, Verrier B, Paul S. (2016) Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials. 75:327-339.
Rochereau N, Pavot V, Verrier B, Ensinas A, Genin C, Corthésy B, Paul S. (2015) Secretory IgA as a vaccine carrier for delivery of HIV antigen to M cells. Eur J Immunol. 45(3):773-9.
Pavot V, Berthet M, Rességuier J, Legaz S, Handké N, Gilbert SC, Paul S, Verrier B. (2014) Poly(lactic acid) and poly(lactic-co-glycolic acid) particles as versatile carrier platforms for vaccine delivery. Nanomedicine (Lond). 9(17):2703-18.
Pavot V, Rochereau N, Rességuier J, Gutjahr A, Genin C, Tiraby G, Perouzel E, Lioux T, Vernejoul F, Verrier B, Paul S. (2014) Cutting edge: New chimeric NOD2/TLR2 adjuvant drastically increases vaccine immunogenicity. J Immunol. 193(12):5781-5.
Collaborations : T. De France, ADITEC members, Adjuvatis, S. Paul, B. Combadière, R. Le Grand, T. VanCott (NIAID), Sanofi Pasteur, GSK, F. Garcia.
Grants : Aditec, Pierre Bergé Sidaction, bioAster, HIVERA, H2020 ; ANRS phD (feb2015-2018), ANR MemoSign.




