Project leader : S. Richard (MCU)
Participants : E. Colomb (IE), A, N. El Kholty (AI), E. Lambert (MCU), D. Le Guellec (PU), M. Lamrayah (Post Doc).
The efficiency of nanovector migration through biological barriers and extracellular matrix toward their targets has been a major obstacle to their development in nanomedecine. To improve the delivery of drug- or antigen-loaded PLA nanovectors, we elaborate multifunctional nanoparticles designed to target and stimulate specific cell sub-populations involved in mucosal immunity. Understanding how nanovectors cross biological boundaries and reach their targets in vivo is key to their development and requires three-dimensional immuno-competent models, able to mimic in vivo conditions. We monitor nanoparticle distribution at the cell and tissue level, and take advantage of various models (mouse, zebrafish and cultures of epithelial and immune cells) to image nanocarrier delivery to specific target cells and subcellular compartments.
> Biodistribution of nanosystems in mammals
Visualization of nanoparticle fate and transport in biological tissues is followed up using in vivo models such as transgenic mice and non human primates (in collaboration with IDMIT, CEA). Using a mouse ligated intestinal loop ex vivo assay, we showed that PLA nanoparticles cross the gut mucosa at the level of Peyer’s patches. After administration of nanoparticles to the living animal, biodistribution is analyzed by live fluorescence tomography, or by histological approaches and confocal microscopy on dissected organs such as skin biopsies or lymphatic nodes.
> Uptake and whole-body biodistribution of nanosystems in zebrafish
A major advantage of zebrafish is ease of imaging at whole-body level. Zebrafish larvae are transparent and the casper mutant line remains semi-transparent at adult stages, which allows their observation by non-invasive fluorescence microscopy. Their small size also facilitates micro-sections of the whole organism, making the assessment of nanovector biodistribution much simpler in zebrafish than in any other vertebrate model. We analyze how tissues respond to nanovectors, which cells take them up and by what mechanisms.
By combining microscopy and flow imaging studies, we showed that PLA nanoparticles cross mucosae at the gill, intestine and skin level (which constitute a mucosa in aquatic organisms) and are efficiently accumulated in mucosal antigen presenting cells (Rességuier et al., Front. Immun. 2017). Using zebrafish from transgenic reporter lines, we screen the in vivo efficiency of various formulations at reaching their target cells and delivering their cargo molecules. We develop technically challenging approaches such as in toto imaging and correlative microscopy. We also use zebrafish as a model for cytotoxic studies of our particulate systems.
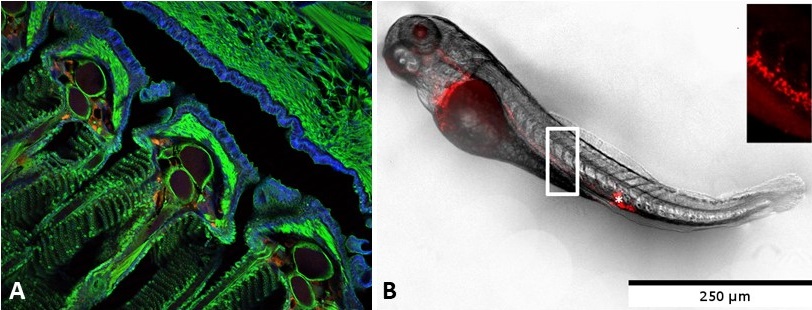
A: Red PLA nanoparticle uptake at gill level after mucosal delivery in adult fish (J. Rességuier, JCR, 2021). B: Biodistribution of red PLA nanoparticles after intravenous injection in a 3 days old zebrafish.
> Use of integrin ligands to increase PLA nanoparticle transport through skin and mucosae, and delivery to subepithelial immune cells
Cell targeting is prompted by a bio-inspired strategy using cellular or viral mimetic approaches and is achieved by addition of specific molecules. For instance, we functionalized nanoparticles with integrin ligands to improve nanoparticle delivery to α5β1 positive cells such as microfold (M) cells of the digestive tract (specialized in antigen transcytosis), dermal dendritic cells, and more generally a wide range of fibronectin-interacting cells in normal or pathological tissues.
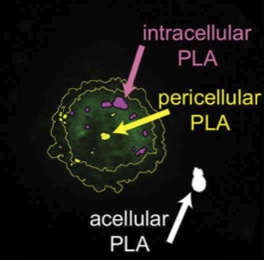
Internalization of integrin ligand-coated PLA nanoparticles in HT1080 cells (Dalzon, Lebas, Gimenez et al., PLOS ONE, 2016).
> Intracellular processing and cytosolic delivery of PLA nanoparticles
It is essential to better understand the fate of nanocarriers and their cargo within cells: do all nanoparticles remain within a cell compartment while their hydrophobic cargo diffuses out into the cytosol? Is there a proportion of intact nanoparticles that is released with their cargo into the cytosol? We analyze these questions using a variety of tracers and flow imaging, laser microscopy and electron microscopy approaches.
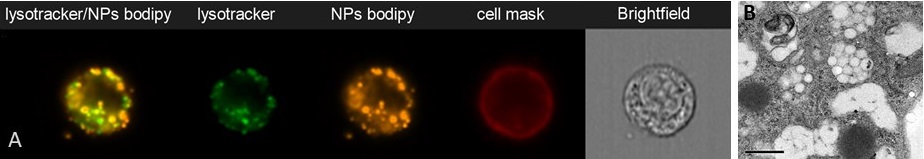
Accumulation of PLA nanoparticles in endosomal compartments of mouse dendritic cells (SRDC), analysez by flow imaging (ImagestreamX, A) (P. Mercier) or TEM (B, Aline et al. 2009)
Selected publications
Rességuier J, Delaune E, Coolen A-L, Levraud J-P, Boudinot P, Le Guellec D and Verrier B (2017) Specific and Efficient Uptake of Surfactant-Free Poly(Lactic Acid) Nanovaccine Vehicles by Mucosal Dendritic Cells in Adult Zebrafish after Bath Immersion. Front. Immunol. 8:190.
Dalzon B, Lebas C, Jimenez G, Gutjahr A, Terrat C, Exposito J-Y, et al. (2016) Poly(Lactic Acid) Nanoparticles Targeting α5β1 Integrin as Vaccine Delivery Vehicle, a Prospective Study. PLoS ONE 11(12): e0167663.
Stability Of Polylactic Acid Particles And Release Of Fluorochromes Upon Topical Application On Human Skin Explants. Liard C, Munier S, Joulin-Giet A, Bonduelle O, Hadam S, Duffy D, Vogt A, Verrier B, Combadière B (2012) Eur J Pharm Biopharm 31 : 6060-8
Rancan F, Todorova A, Hadam S, Papakostas D, Luciani E, Graf C, Gernert U, Ruhl E, Verrier B, Sterry W, Blume-Peytavi U, VogtA (2012). Intradermal immunization triggers epidermal Langerhans cell mobilization required for CD8 T-cell immune responses. J Invest Dermatol 132(3 Pt 1):615-25
Liard C, Munier S, Joulin-Giet A, Bonduelle O, Hadam S, Duffy D, Vogt A, Verrier B, Combadière B(2012). Stability Of Polylactic Acid Particles And Release Of Fluorochromes Upon Topical Application On Human Skin Explants. Eur J Pharm Biopharm 31 : 6060-8
Primard C, Rochereau N, Luciani E, Genin C, Delair T, Paul S, Verrier B (2010). Traffic Of Poly(lactic Acid) Nanoparticulate Vaccine Vehicle From Intestinal Mucus To Sub-epithelial Immune Competent Cells. Biomaterials 31 : 6060-8
Aline F, Brand D, Pierre J, Roingeard P, Munier S, Verrier B, Dimier-Poisson I (2009). Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic PLA nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine 27(38):5284-91.
Collaborations:
UMS 3444 (ImageStreamX, Cytof, PRECI, PLATIM)
Centre d’imagerie des microstructures
Dr. J.P. Levraud (Pasteur Institute, Paris)
Dr. P. Boudinot (INRA, Jouy en Josas),
Dr. J.S. Joly (CNRS, Gif-sur-Yvette)
Dr. P. Affaticati (Tefor Core Facility, Gif-sur-Yvette)
Grants: PECSDDeli, BQR, ADITEC, Fish-RNAvax




