Project leader : F. Mallein-Gerin
Participants : Maxime Ducret, Emeline Perrier-Groult, Marielle Pasdeloup
The interest in cell therapy and tissue engineering for cartilage repair is increasing and actually, autologous chondrocytes represent the most common source of cells that are transplanted. The use of mesenchymal stem cells (MSC) represents a promising alternative cellular model but satisfactory protocols allowing their chondrogenic conversion together with sufficient cartilage matrix production are still missing. The MSC are found in a variety of tissues and are already used to induce cell differentiation, but with variable efficacy depending on their origin and the lineage considered. Moreover, it is difficult to rank the origin of the MSC in regard of their potential of proliferation and differentiation, from the studies published in the literature. The inter-donor variability and the differences in cell culture conditions used by the research groups hamper a clear identification of subpopulations that could represent precursors of interest for a specific differentiation lineage.
In this context, we have undertaken a comparative flow cytometry analysis of MSC isolated from Wharton’s jelly, adipose tissue, dental pulp, and bone marrow, the gold-standard for MSC (figure 1). An original panel of 27 surface markers has been selected from the literature. The different categories of MSC are cultured in medium without serum, condition allowing more easily their use in clinical application. Very likely, the co-expression of several markers will allow identification of subpopulations of interest. For instance, we have observed for the first time combined labeling of CD271/CD56/Stro-1, surface markers that are independently proposed as predictive markers for good proliferation, immunosuppression or ability to engage skeletogenesis. We have registered significative differences between the diverse categories of MSC. Such analysis will be pursued and selected sub-populations will be evaluated for cell proliferation and differentiation, and immunosuppressive properties. Special efforts are made to identify the best source of MSC and culture conditions for efficient chondrogenic differentiation.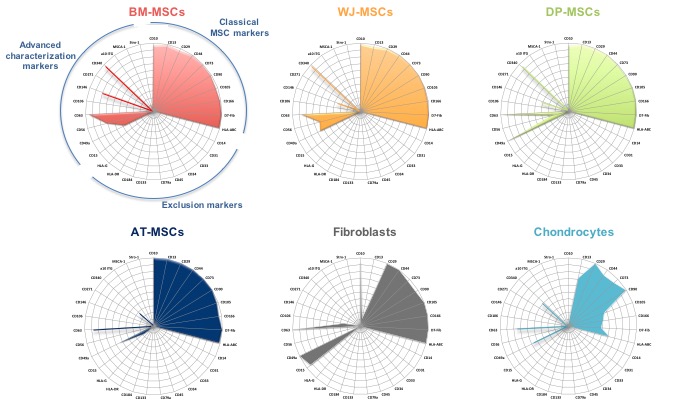
Figure 1 : original polychromatic analysis of 31 surface markers of MSCs (bone marrow, perinatal derived tissue from Wharton jelly, dental pulp, and adipose tissue) or fibroblasts and chondrocytes cultured without serum (flow cytometry). The sonar illustration show the percentage of expression (3 to 8 donors).
Selected publications :
– Fabre H, Ducret M, Degoul O, Rodriguez J, Perrier-Groult E, Aubert-Foucher E, Pasdeloup M, Auxenfans C, McGuckin C, Forraz N, and Mallein-Gerin F. Characterization of Different Sources of Human MSCs Expanded in Serum-Free Conditions with Quantification of Chondrogenic Induction in 3D. Stem Cells International. Article ID 2186728, Volume 2019 (2019)
– Ducret M, Farges JC, Pasdeloup M, Perrier-Groult E, Mueller A, Mallein-Gerin F, Fabre H. Phenotypic Identification of Dental Pulp Mesenchymal Stem/Stromal Cells Subpopulations with Multiparametric Flow Cytometry. Methods Mol Biol. 2019;1922:77-90. doi: 10.1007/978-1-4939-9012-2_8.
– Rodriguez J, Pratta AS, Abbassi N, Fabre H, Rodriguez F, Debard C, Adobati J, Boucher F, Mallein-Gerin F, Auxenfans C, Damour O, Mojallal A. Evaluation of Three Devices for the Isolation of the Stromal Vascular Fraction from Adipose Tissue and for ASC Culture: A Comparative Study. Stem Cells Int. 2017;2017:9289213. doi: 10.1155/2017/9289213.
– Ducret M., Fabre H., Farges J.-C., Degoul O., Atzeni G., McGuckin C., Forraz N., Malleing-Gerin F., Perrier-Groult E.
Production of human dental pulp cells with a medicinal manufacturing approach. J. Endod. 2015 ; 41(9):1492-9 doi : 10.1016/j.joen.2015.05.017
– Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, Lafont J, Denoix JM, Audigié F, Mallein-Gerin F, Legendre F, Galera P. (2014). Cartilage tissue engineering : molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction Biochim Biophys Acta. (general subjects) S0304-4165(14)00091-9
Collaborations :
O. Damour, Laboratoire des Substituts Cutanés et Banque de Tissus et de Cellules, Hôpital Edouard Herriot, Lyon
N. Forraz et C. McGuckin, CTI-BIOTECH, Lyon




